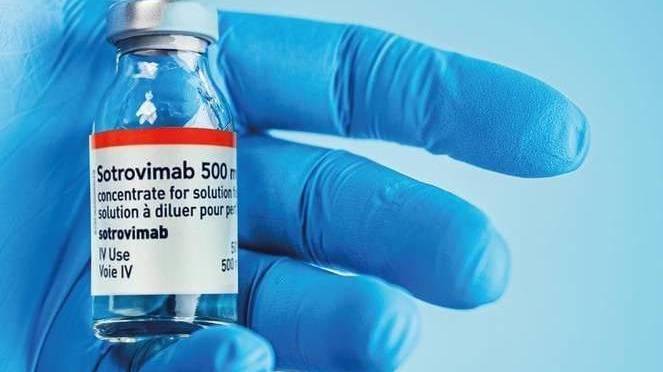
The Federal Government has secured an initial shipment of over 7,700 doses of a breakthrough new drug for the treatment of coronavirus.
GlaxoSmithKline’s (GSK) novel monoclonal antibody treatment ‘Sotrovimab’ has been shown to dramatically reduce hospitalisation and risk of death in adults with mild to moderate COVID-19, who are at risk of developing severe COVID-19.
To date, Sotrovimab has been shown to reduce hospitalisation or death by 79% in adults with mild to moderate COVID-19 who are at high risk of progression to severe disease.
“Sotrovimab will provide another tool in the ongoing challenge against COVID-19. It will provide an important new way to treat the disease and manage outbreaks across Australia,” said Federal Health Minister, Greg Hunt.
Sotrovimab is currently being assessed by the Therapeutic Goods Administration (TGA) and is expected to be available for use in Australia this year once the TGA evaluations are complete.
The Government’s advanced purchase of sotrovimab has been supported by the Science and Industry Technical Advisory Group (SITAG), which is the Australian Government’s expert group advising on COVID-19 vaccine and treatment purchases.
“Antibodies are proteins produced by our own body’s immune system and are one of the main ways the body defends itself against diseases. Antibodies work by binding to a specific target – for example, a virus or a bacteria – and making them harmless. They block or slow down the action of the virus or bacteria, or they flag it as ‘foreign’ so that other parts of our immune system can clear the ‘invaders’ away,” said Mr Hunt.
“Monoclonal antibodies work in the same way. They are laboratory-made proteins that mimic our body’s immune system to help fight off harmful pathogens and can be used to help to treat people who already have COVID-19.
“Monoclonal antibodies have been safely and effectively used to treat a growing number of diseases, some of which were difficult to treat in the past.”
Manufactured by GSK, Sotrovimab will be the first COVID-19 monoclonal antibody treatment available for use in Australia, with the complete treatment requiring just one dose administered via IV infusion in a healthcare facility.
Sotrovimab has been used successfully in the United Arab Emirates, where health authorities this month announced that the drug had led to the recovery of 97% of patients over a two-week period.
It has also been trialled on volunteers in the US, Canada, Brazil and Spain. The US Food and Drug Administration placed an order for 220,000 doses in May.
It won’t be plain sailing for the drug on our shores though, with the National COVID-19 Clinical Evidence Taskforce recommending the drug should not yet be used to treat COVID-19 outside of clinical trials.
Taskforce director, Professor Julian Elliott said there was not enough high-quality evidence to prove the drug worked.
The Australian Government’s agreement with Sotrovimab’s supplier includes delivery of more than 7,700 doses for the National Medical Stockpile, with an initial delivery this year upon TGA approval.
“As with all products procured for the National Medical Stockpile, this treatment will be provided to states and territories as needed, to be administered to eligible patients in a healthcare facility,” Mr Hunt siad.
“Not all people with COVID-19 will require access to this treatment. Where a doctor prescribes this treatment for their patients with mild to moderate COVID-19, who are at risk of developing severe COVID-19, it will be made available free of charge through the public health system.
“Physician estimates of the Australian patient treatment population range from eight to fifteen per cent of patients who are SARS-CoV2 positive that would be considered at high risk of disease progression and would be recommended for treatment with Sotrovimab, based on past and current experience of managing COVID-19 patients.”