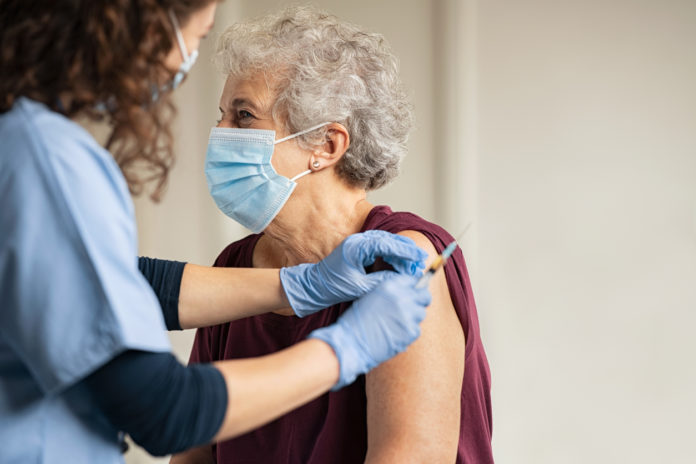
Volunteers are being sought for a world-first trial to establish the efficacy of giving people a first dose of one vaccine and a second dose of a different vaccine.
The trial, which is being run by Oxford University and is funded by the government’s vaccine taskforce, has been described by ministers as “hugely important”.
It will recruit 820 people over the age of 50 who have not yet had a vaccine, to receive a first dose of either the Oxford/AstraZeneca vaccine or the Pfizer/BioNTech vaccine. Some people will then get an alternative vaccine at a second appointment within 12 weeks, and others will get the same vaccine again.
Scientists want to know whether protection from mixing vaccines is the same, reduced or even better, compared with adhering to the same vaccine throughout.
With the steady supply of vaccines always in question, the researchers said the information they collect would be useful not only for the UK but for the whole world.
There is also the possibility that giving an individual two different vaccines in a row might give greater protection against the more infectious Covid variants that have emerged in the UK, South Africa and Brazil.
“If we do show that these vaccines can be used interchangeably in the same schedule, this will greatly increase the flexibility of vaccine delivery and could provide clues as to how to increase the breadth of protection against new virus strains,” said Matthew Snape, an associate professor in paediatrics and vaccinology at the University of Oxford, and the chief investigator in the trial.
At a briefing, Snape said there was evidence from mice studies that combining an adenoviral vector vaccine, such as the Oxford jab, with an mRNA vaccine, such as Pfizer/BioNTech’s, could generate a better response.
Both vaccines – and the Novovax and Janssen vaccines, which are likely to be added to the trial if approved – target the spike protein of the virus. “For that reason I think we do anticipate that will generate a good immune response with these combinations, but we have to test it first, we have to see,” he said.
Volunteers, who will be recruited through the NHS vaccine research volunteer website, will have blood taken to measure the buildup of antibodies after vaccination against the virus. Some will have their second dose after four weeks’ time, and some after a 12-week interval, to provide more data on the government policy of widening the dosage gap.
Prof Jonathan Van-Tam, the deputy chief medical officer for England and the senior responsible officer for the study, said: “Given the inevitable challenges of immunising large numbers of the population against Covid-19 and potential global supply constraints, there are definite advantages to having data that could support a more flexible immunisation programme, if needed and if approved by the medicines regulator.
“It is also even possible that by combining vaccines the immune response could be enhanced, giving even higher antibody levels that last longer. Unless this is evaluated in a clinical trial, we just won’t know. This study will give us greater insight into how we can use vaccines to stay on top of this nasty disease.”
Nadhim Zahawi, the minister for Covid-19 vaccine deployment, called it “a hugely important clinical trial that will provide us with more vital evidence on the safety of these vaccines when used in different ways”.
He said mixing vaccines would not be recommended outside the study “until researchers and the regulator are absolutely confident the approach is safe and effective”.
This article first appeared in The Guardian.