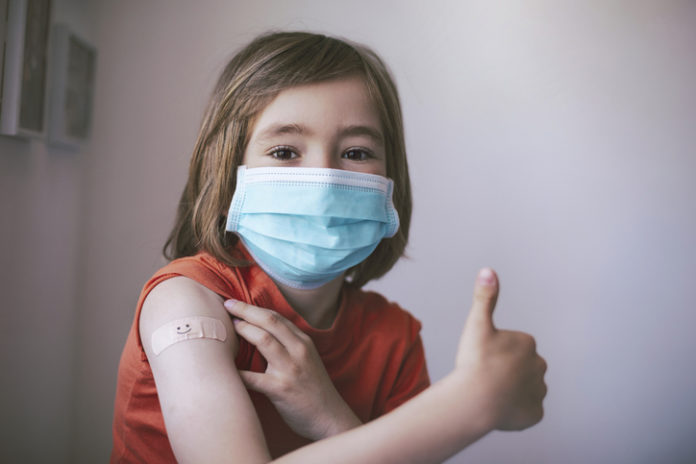
Vaccinating Australian children aged 5 to 11 years old against COVID-19 is a significant step closer, with the Therapeutic Goods Administration (TGA) provisionally approving the Pfizer vaccine as safe and effective for use for young children.
Subject to final considerations and recommendations from the vaccination experts on the Australian Technical Advisory Group on Immunisation (ATAGI), and other related approvals, the Australian Government says it will start rolling out the vaccine to 5 to 11-year-olds from 10 January next year.
“The Government expects to receive ATAGI’s recommendations on how to incorporate this safe and effective vaccine into Australia’s COVID-19 vaccination program over the coming weeks,” Federal Health Minister, Greg Hunt said in a statement today.
Subject to advice from ATAGI, vaccinating the approximately 2.3 million children aged 5 to 11 in Australia will build on the rapid uptake of vaccination among children aged 12 to 15. In just eleven weeks, more than 76.6% of this group have had at least one dose of vaccine, with 67.5% having completed their two-dose course of vaccination, the health minister said.
Across the country, 87.9% of Australians aged 16 or over are fully vaccinated. More than 92.8% have had at least one dose of COVID-19 vaccine.
“The TGA’s provisional approval of the Pfizer vaccine for 5 to 11-year-olds was based on a careful evaluation of available data to support its safety and efficacy among this age group,” said Minister Hunt.
“The vaccine dose approved by the TGA for children aged 5 to 11 is the same safe and effective vaccine used for other age cohorts, however is one-third the dose approved for those aged 12 and over.”
As with other age groups, the use of this vaccine in children aged 5-11 years should be given in two doses at least three weeks apart, he said.
The first shipment of children’s doses are due to arrive in Australia by early January 2022 and will undergo the same rigorous batch testing processes in the TGA laboratories as other batches of COVID-19 vaccines, Mr Hunt said.
The TGA is also currently evaluating an application from Moderna for its COVID-19 vaccine to be used in Australia for children aged 6 to 11 and the Government already has supply deals in place to make it available should it be approved by the TGA and recommended by ATAGI.