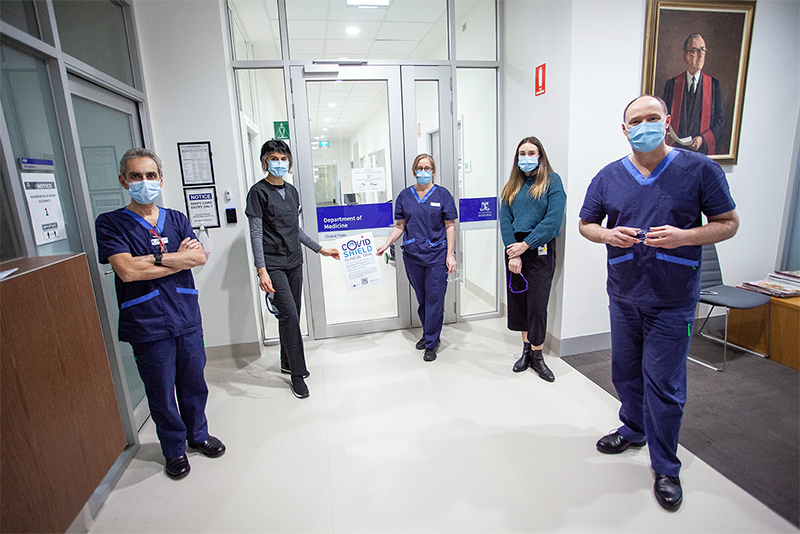
The St Vincent’s trial team: Prof John Santamaria (ICU), A/Prof Mandana Nikpour [Rheumatology], Jennifer Holmes [ICU], Dr Katherine Ellis [Rheumatology] and Roger Smith [ICU]
Melbourne’s St Vincent’s Hospital is set to trial the controversial drug that US President Donald Trump has publicly paraded as his own personal treatment for coronavirus.
The hospital has advertised to recruit up to 650 doctors and nurses to take part in the trial, which will see half of the participants take the controversial drug, hydroxychloroquine, with the remainder taking a placebo.

It’s hoped the COVID SHIELD Clinical Trial – being led by the Walter and Eliza Hall Institute of Medical Research – will help to determine whether the drug could provide protection for healthcare workers treating COVID-positive patients.
“For every healthcare worker who is infected, quite a few then have to be tested, or go into self-isolation for two weeks,” said Associate Professor, Mandana Nikpour, one of COVID SHIELD’s lead principal researchers and a consultant rheumatologist at St Vincent’s Hospital.
“This has had an impact on the physical and mental health of healthcare workers, as well as an impact on the workforce we have available to undertake frontline duties.
“For every healthcare worker who is infected, quite a few then have to be tested, or go into self-isolation for two weeks. This has had an impact on the physical and mental health of healthcare workers, as well as an impact on the workforce we have available to undertake frontline duties,” A/Prof Nikpour said.
Hydroxychloroquine is commonly used in the treatment of lupus and rheumatoid arthritis.
Having used the drug to successfully treat patients with both these conditions, A/Prof Nikpour says initial findings indicate hydroxychloroquine could potentially help protect healthcare sector workers from the deadly virus.
“In the test-tube, hydroxychloroquine reduces the replication of SARS Coronavirus 2, which is the COVID-19 virus, by about 90 percent,” she explained.
“When you are very sick or ventilated in hospital you have a lot of inflammatory molecules causing collateral damage to organs, and it is not possible at that point to intervene with a drug like hydroxychloroquine. However, hydroxychloroquine could potentially reduce the risk of COVID-19, if taken prior to exposure to the virus, that is, as ‘pre-exposure prophylaxis’ or ‘PrEP’.”
Back in March the Therapeutic Goods Administration (TGA) raised its concerns about the potential use of the drug to treat coronavirus here in Australia.
“Clinical trials are underway around the world examining their potential to treat COVID-19,” the TGA said in a statement.
“However, these medicines pose well-known serious risks to patients including cardiac toxicity (potentially leading to sudden heart attacks), irreversible eye damage and severe depletion of blood sugar (potentially leading to coma).
“Given the limited evidence for effect against COVID-19, as well as the risk of significant adverse effects, the TGA strongly discourages the use of hydroxychloroquine outside of its current indications at this time other than in a clinical trial setting or in a controlled environment in the treatment of severely ill patients in hospital.”
Around 40 healthcare workers have so far signed up for the trial.